Researchers have developed a highly robust gel that includes large amounts of ionic liquid. The research team was led by Professor MATSUYAMA Hideto and Assistant Professor KAMIO Eiji (Kobe University Graduate School of Science, Center for Membrane and Film Technology). These findings were published on November 8 in Advanced Materials.
Ionic liquid is a substance made solely from ions, and it has unique properties – for example, it does not evaporate at normal temperatures or pressures, and it has high thermal stability. Gels that contain ionic liquid are known as ion gels. With the same properties as ionic liquids, as well as their ability to retain liquid form, they can potentially be used as electrolytes for rechargeable batteries and as membranes for gas separation. However, the low mechanical strength of typical ion gels limits their practical applications.
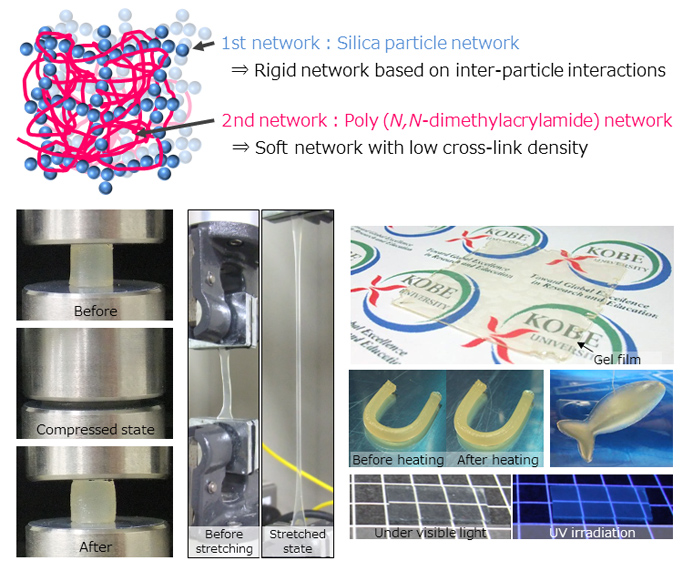
The research team created a double network within ionic liquid, combining a network of inorganic silica particles with a network of organic polymers. This dramatically improved the resilience of the ion gel, and the gel that they developed can withstand more than 25 MPa of compressive strength without breaking. The strength of the newly-developed robust ion gel originates in the special interpenetrating double network. When stress is applied to the gel, the brittle silica particle network breaks and dissipates the loaded energy. The physical interaction between the silica particles enables the network to self-recover. Most of the ionic liquid contained in the gel does not vaporize, so it can be stored in a stable condition for a long time. Even exposing it to a high temperature vacuum does not damage its performance, so it can also be used in high temperature fields.
The gel obtained from this research could be used in CO2 separation membranes or as electrolytes for rechargeable batteries. Our research team will collaborate with businesses to find practical applications for this gel. They will also continue to analyze the strengthening mechanism in more detail, and aim for a higher-performance, stronger gel by designing the perfect network.
Journal information
- Title
- “Inorganic/Organic Double-Network Gels Containing Ionic Liquids”
- DOI
- 10.1002/adma.201704118
- Authors
- E. Kamio, T. Yasui, Y. Iida, J. P. Gong and H. Matsuyama
- Journal
- Advanced Materials